Introduction: Teclistamab (Tec) is a T-cell redirecting bispecific antibody targeting CD3 on T-cells and B-cell maturation antigen on plasma cells. It has demonstrated a high rate of deep responses in patients with heavily pre-treated multiple myeloma in the MajesTec-1 study, gaining FDA approval in October 2022. However, patients with amyloidosis were excluded from the MajesTec-1, so the safety and efficacy of Tec in patients with AL amyloidosis is not currently known.
Methods: We retrospectively analyzed adult patients with biopsy-proven AL amyloidosis that were treated with Tec 12/2022-7/2023 at the Hospital of the University of Pennsylvania. The revised Mayo Clinic criteria were used for staging. Adverse events (AE) were extracted from the electronic medical record. Patients were assessed for hematologic and organ response per consensus guidelines.
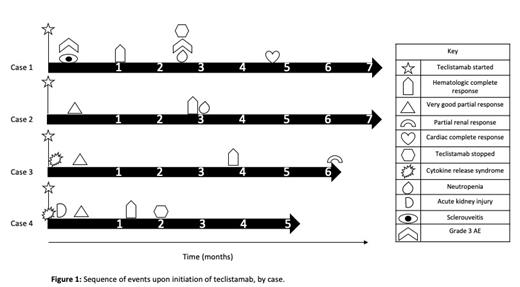
Results: Four patients were included in this case series (Figure 1). The median age was 63 at time of Tec (range 59-65), 3/4 were female, and all had multi-organ involvement of amyloidosis, including 3/4 with cardiac (2 Stage III, 1 Stage IV), 4/4 with renal, 2/4 with gastrointestinal involvement, and 3/4 with subcutaneous involvement. All patients also met IMWG criteria for multiple myeloma. The median number of prior lines of therapy was 7, including cyclophosphamide, bortezomib, daratumumab, lenalidomide in all patients and pomalidomide in 3/4, elotuzumab in 2/4, carfilzomib in 2/4, bendamustine in 2/4, venetoclax in 1/4, and belantamab-mafodotin in 1/4 . Two patientshad previously underwent autologous stem cell transplant. The ORR was 100%, with all four patients eventually achieving hematologic complete response (hCR). The median time to hematologic very good partial response (hVGPR) was 12 days (range 11-12 days) and hCR was 62 days (range 32-117 days). One patient achieved a cardiac response at 144 days and another achieved a renal response at 191 days. All patients were still alive and in continued hCR at the time of this report, with a median duration of follow-up of 210 days (range 173-215 days) and a median completion of 4.5 cycles of Tec (range 2-7). Regarding safety, 2/4 patients developed cytokine release syndrome, 1 Grade I on day of first priming dose and the other Grade II on date of second priming dose, both of which lasted one day and improved after tociluzumab administration. No patients developed ICANS. Two patients had treatment-emergent neutropenia occurring several cycles into therapy, with one presenting as agranulocytosis that improved with G-CSF and treatment discontinuation. Additional non-hematologic AE of note included one patient developing Grade 1 acute kidney injury and one developing Grade 3 sclerouveitis, all during the first 5 days of exposure. No patients developed cardiac decompensation while on therapy.
Conclusion: In this series of heavily pre-treated patients, Tec showed outstanding depth of response with all patients achieving hCR (FLC-diff <10 mg/L and negative serum/urine immunofixation), associated with organ responses in 2/4 patients (Figure 1). Tec was well-tolerated, with cytokine release syndrome being manageable in this population, though acute kidney injury and treatment-limiting neutropenia did occur. Teclistamab shows promise in treating patients with relapsed AL amyloidosis, but further prospective studies and longer duration of follow-up are needed to determine long-term outcomes and to optimize safety in this at-risk patient population.
Disclosures
Garfall:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring Board; BMS: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Legend: Consultancy, Honoraria. Cohen:BMS/Celgene: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Abbvie: Consultancy; Pfizer: Consultancy; Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Arcellx: Consultancy. Vogl:GSK: Consultancy; Active Biotech: Research Funding; Karyopharm: Consultancy; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Genentech: Consultancy. Stadtmauer:BMS: Consultancy; Janssen: Consultancy; Abbvie: Consultancy, Research Funding; Amgen: Consultancy; genmab: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal